THE DRUG MANUFACTURING PROCESS : FROM RESEARCH TO MARKET LAUNCH
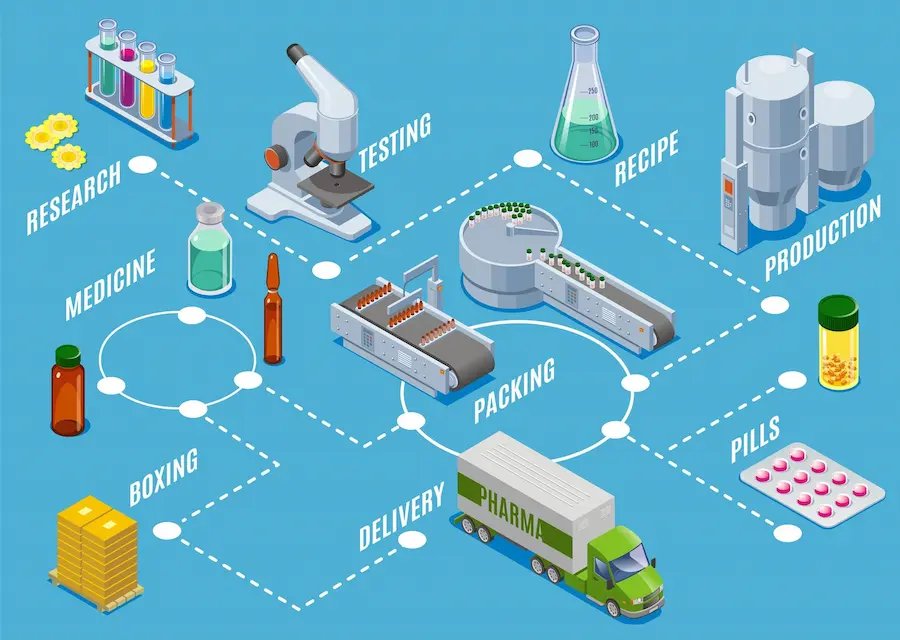
The pharmaceutical industry is a complex field in which the research, development and marketing of a medicine follow a rigorous path. There are many stages in this circuit , all designed to ensure the efficacy, safety and quality of the products that reach patients. Let’s take a look at how the drug manufacturing works, from conception to availability on pharmacy shelves.
Research and testing: 8 to 10 years
The first stage in the development of a drug is pharmaceutical research. It often begins with the identification of unmet medical needs in the treatment of a specific disease. Once these needs have been clearly defined, scientists embark on a relentless quest to discover new molecules or develop innovative treatments.
Patent registration
The research process can take years, and each discovery goes through rigorous stages. First, a patent is filed to protect the new compound or technology developed. Next, preclinical trials are carried out to assess the drug’s toxicity, pharmacokinetics and potential efficacy in animal models.
Clinical tests
Once pre-clinical results are satisfactory, drug candidates are selected, followed by clinical trials. These tests on humans are conducted in several phases to assess the efficacy and safety of the drug in patients. This stage can take several years and involve thousands of participants.
Approvals and production start-up: 1 to 3 years
Once the clinical trials have been completed and the results analysed, the drug manufacturer must obtain regulatory authorisation to market the product. The Marketing Authorisation Application (MAA) is submitted to the relevant authorities, such as the European Medicines Agency (EMA) or the Food and Drug Administration (FDA).
Validation of the marketing authorisation dossier requires a full presentation of research data, the results of clinical trials, and information on the manufacture and quality control of the drug. Transparency is essential at this stage, with open communication on the risks and benefits of the drug.
Once marketing authorisation has been obtained, the price and reimbursement of the drug are set, often in collaboration with public health bodies and insurance companies. The industrial manufacture of the drug then begins, following Good Manufacturing Practices (GMP) to guarantee its quality, safety and efficacy throughout the production process.
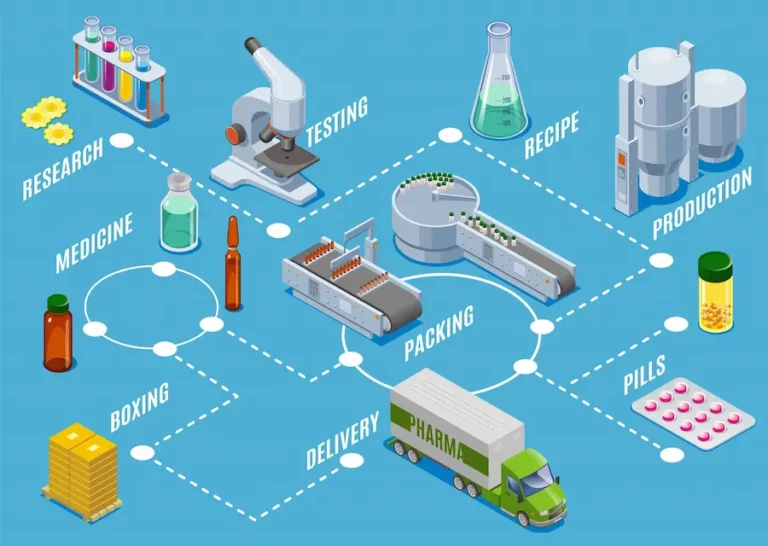
Life of Medicines: 20 years and over
A medicine begins its active life as soon as it is made available on the market. For approximately 20 years, the patent holder has exclusive marketing rights. It is only once this period has elapsed that the property right expires. From then on, other manufacturers can produce generic versions of the drug.
The end of the patent often marks the start of a period of increased competition and lower prices, which can benefit patients by making treatments more accessible. However, generic manufacturers must also comply with strict standards, while offering effective alternatives to branded products.
This complex journey requires an unwavering commitment to science, regulation and, above all, the health and well-being of patients. Every step of the way must ensure that the medicines that reach their hands are safe, effective and of high quality.